
Rapid, Automated Reporting System for your GLP Bioanalysis Lab
Processing Clinical Data
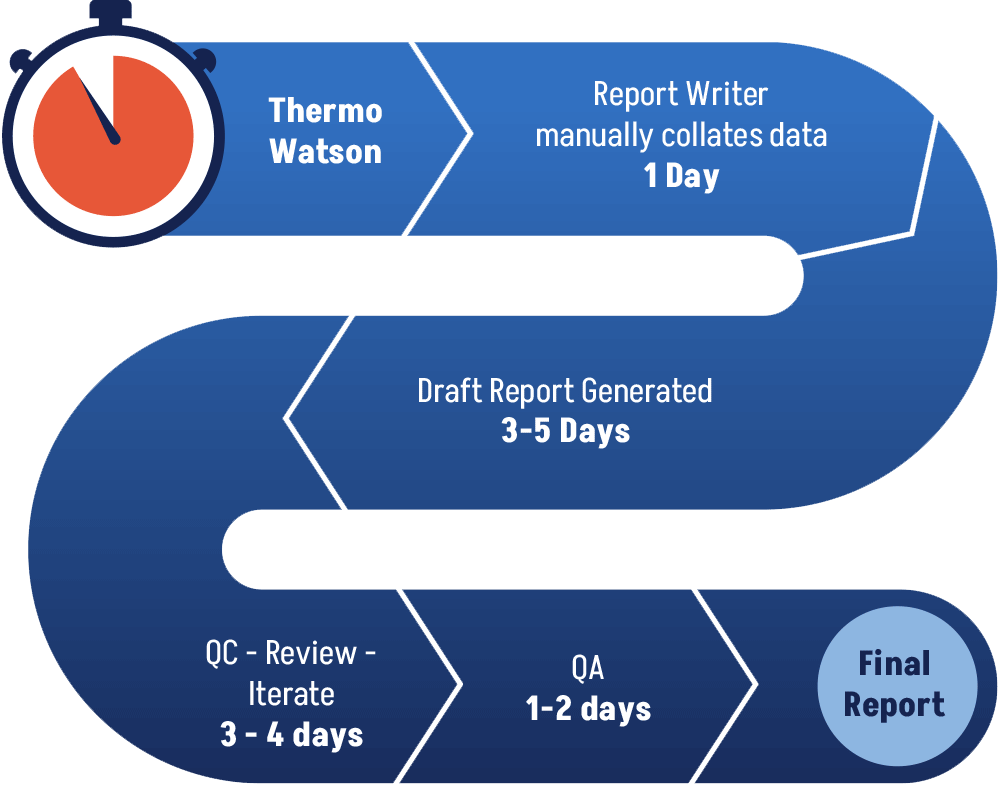
Problem – Current Day
Today’s GLP Method Validation and Bioanalysis Reports generation workflow relies heavily on manual copying/pasting/formatting/
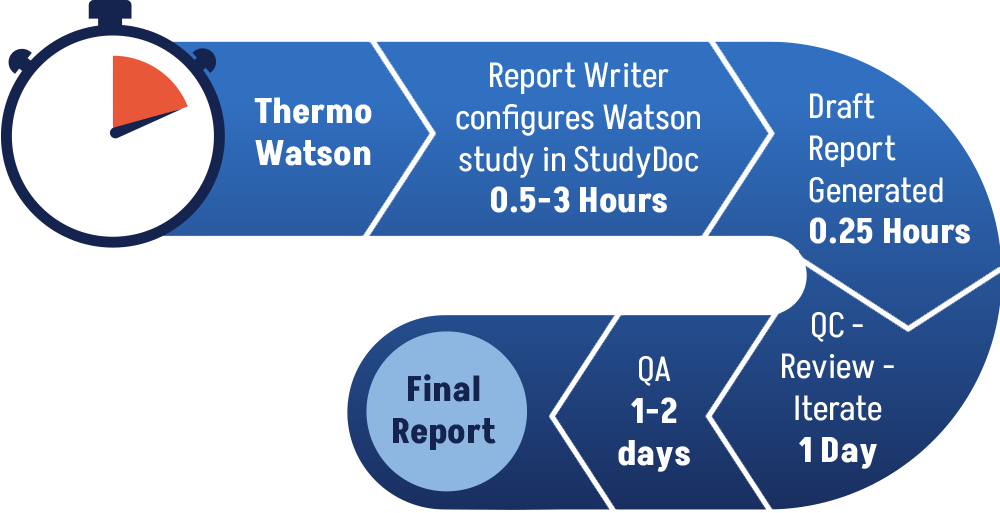
Solution – StudyDoc™
StudyDoc retrieves all study- and table-related raw data from the Thermo Scientfic Watson database. This allows labs to almost immediately produce a consistently formatted, hyperlinked, and accurate draft GLP Method Validation and Bioanalysis Reports upon study completion meaning Final Reports can be issued more quickly. This also allows labs to complete more studies in the same given time frame.
Automated
Seamlessly assign and compile report data from Thermo Scientific Watson database.
Adaptable
GLP Method Validation and Bioanalysis Reports created from user-provided Microsoft Word templates. StudyDoc field codes allow a single template to be used for single- or multi-analyte studies. Unlimited number of templates allowed.
Compliant
Provides full or partial 21 CFR Part 11 support (security, electronic signatures, and audit trails).
Save Time + Money
Cut unnecessary cost by reducing manual processes, minimizing chance of human error, and eliminating redundant review.
StudyDoc by LabIntegrity understands the complex and critical nature of this industry and how important it is to provide accurate reporting in an efficient manner. Throughout our careers in this industry, we have experienced the shortcomings of traditional report writing first hand. Given our experience throughout the years, we are now in a position to completely optimize how report writing is conducted.
StudyDoc Features
StudyDoc is a robust data reporting software that is built to fit your needs. Check out a few of our main features and click to see just how StudyDoc will improve your process.
Regulatory Support
Complete support for GLP/ICH Small Molecule and Large Molecule assay table formats, including China regulatory body NMPA Small Molecule table formats
Application Portability
Sponsors can send clients StudyDoc Word Templates and StudyDoc Study Templates to configure in the client StudyDoc system, ensuring that client reports are exactly as defined by sponsor.
Field Code Generator
Provides complete control over how the text sections of reports are generated such that the complete section of tables, any individual table, or any table- specific statistical data can be put anywhere in the final document template. Specific references to tables in the form of hyper-links are also available.
Document Content Management and Version Control
All document modifications are version controlled. Users may compare versions of documents, make modifications as necessary and save as a new version.
21 CFR Part 11 Compliance
Audit trails keep track of any changes made in the report generation process. Electronic signatures allow the software to restrict any fraudulent StudyDoc changes. The compliance feature can be configured as a silent audit trail without an electronic signature prompt.
